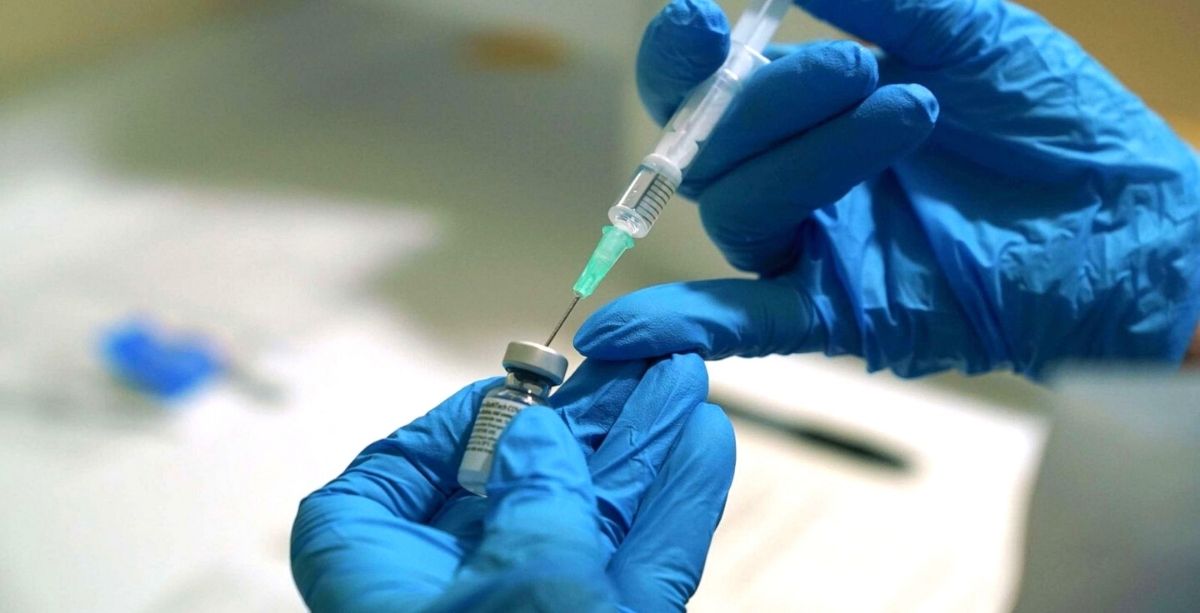
The United States Food and Drugs Administration (FDA) has issued an emergency use authorization for the coronavirus (COVID-19) vaccine developed by Pfizer and BioNTech.
This is the first authorization for a COVID-19 vaccine in the U.S., and it allows the drug to be distributed in the country. It was approved for the vaccination of individuals aged 16 years and older.
“The FDA’s authorization for emergency use of the first COVID-19 vaccine is a significant milestone in battling this devastating pandemic that has affected so many families in the United States and around the world,” FDA Commissioner Dr. Stephen Hahn said in a statement.
Commenting on the news, incumbent U.S. President Donald Trump called the Pfizer vaccine “one of the greatest scientific accomplishments in history.” He confirmed that the first dose of the vaccine would be administered in less than 24 hours.
Notably, Lebanon has reserved 2 million doses of the 2-dose vaccine and expects its arrival by late February.
However, logistically speaking, its distribution is expected to be one of the most complex public health campaigns in history, as it requires a temperature of 70 degrees Celsius below zero for storage.
This has already posed a challenge locally in the U.S., as Congress has not provided the needed funding for the distribution, U.S. media reported. This consequently left public health authorities on their own to tackle the distribution process.
We have a dedicated coronavirus section where you can find the latest news/updates about the pandemic in Lebanon, inform yourself with WHO-verified resources, and track the number of cases in Lebanon in real-time. Click here.